 Suppose the atoms of a crystal structure have three bonds to three
equally spaced coplanar neighbors. The resulting structure looks like
the picture to the left. In his book Three-Dimensional Nets
and Polyhedra, A.F. Wells suggests that Hydrogen Peroxide,
among other chemicals, will have this crystal structure.
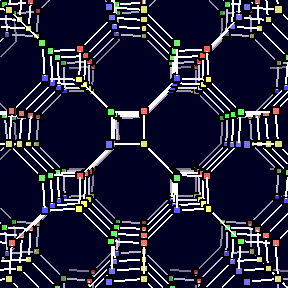
Suppose the atoms of a crystal structure have three bonds to three
equally spaced coplanar neighbors. The resulting structure looks like
the picture to the left. In his book Three-Dimensional Nets
and Polyhedra, A.F. Wells suggests that Hydrogen Peroxide,
among other chemicals, will have this crystal structure.
In 1977, Branko Grunbaum showed that this structure can also be viewed as a polyhedron. The vertices and edges of the structure correspond to atoms and bonds. The faces are the infinite square spirals receding from the viewer in the picture above. If you download and play the mpeg movie of the structure by clicking on the picture, you will also see the receding triangular sprials which are the faces of the Petrie dual of the uniform net.
![[HOME]](/pix/home.gif) The Geometry Center Home Page
The Geometry Center Home Page
Comments to burgiel@geom.umn.edu
Copyright © 1996 by
The Geometry Center.
All rights reserved.